Hongos endófitos aislados de manglares en la Reserva Natural San Pedro, Buenaventura
DOI:
https://doi.org/10.25268/bimc.invemar.2023.52.2.1231Palabras clave:
Análisis molecular, asociación hongo-huésped, ecosistemas costeros, patógenos fúngicos.Resumen
Los árboles de mangle crecen en zonas estuarinas de las regiones tropicales y subtropicales, donde prestan importantes servicios económicos, ecológicos y culturales. Los estudios han demostrado que estos árboles son importantes reservorios de microorganismos fúngicos, que comprenden una serie de hongos morfológicamente diversos incluyendo patógenos, endófitos o saprobios,
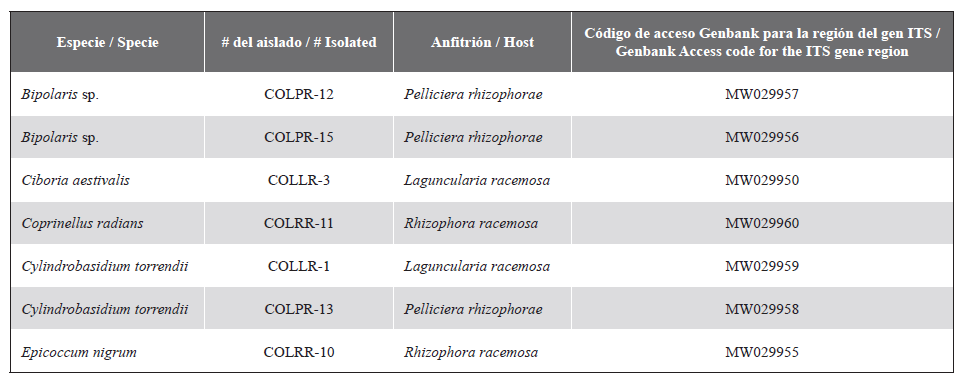
desempeñando un papel clave en el ciclado de nutrientes, protección del hospedero frente a condiciones adversas y, en muchas ocasiones, el declive de los hospederos. Con el fin de determinar la diversidad de hongos endófitos asociados a árboles de mangle en la Reserva Natural San Pedro, Buenaventura, Colombia; se colectaron ramas sanas (longitud aprox. 10 cm) de Laguncularia racemosa, Mora oleifera, Pelliciera rhizophorae y Rhizophora racemosa para los respectivos aislamientos fúngicos. Se extrajo ADN y se amplificó la región del espaciador transcrito interno (ITS), seguido de análisis filogenéticos tales como Bayesiano (BI), Máxima Verosimilitud (ML) y Máxima Parsimonia (MP). En total se identificaron nueve géneros, Bipolaris, Ciboria, Coprinellus, Cylindrobasidium, Epicoccum, Fusarium,
Lasiodiplodia, Neofusicoccum y Neurospora pertenecientes a ocho familias. De estos, Fusarium, Lasiodiplodia y Neofusicoccum son bien conocidos por su alto potencial para amenazar la salud de sus huéspedes cuando sus condiciones son adversas, igualmente los hongos
endófitos también cumplen un papel importante en la producción de biocompuestos para la protección de sus hospedaros.
Citas
Abdel-Wahab, M.A. 2005. Diversity of marine fungi from Egyptian Red Sea mangroves. Botanica Marina, 48: 348–355. doi: 10.1515/BOT.2005.047
Abdel, M.A., M.S. Hodhod, A.H., Bahkali, and E.B. Jones. 2014. Marine fungi of Saudi Arabia. Botanica Marina., 57: 323–335. doi: 10.1515/bot-2014-0010
Agrios, G.N. 2005. Plant Pathology. 5th edn. London: Elsevier/Academic Press
Ahumada, R., V. Novoa, and J. Becerra. 2018. Morphological response to salinity, temperature, and pH changes by marine fungus Epicoccum nigrum. Environmental Monitoring and Assessment, 191. doi: 10.1007/s10661-018-7166-5. PMID: 30593600
Akaike, H. 1974. A new look at the statistical model identification. IEEE Transactions on Automatic Control, AC19: 716–723. doi: 10.1109/TAC.1974.1100705
Alias, S.A., N. Zainuddin, and E.B. Jones. 2010. Biodiversity of marine fungi in Malaysian mangroves. Botanica Marina, 53: 545–54. doi: 10.1515/bot.2010.066
Altschul, S.F., W. Gish, W. Miller, E.W. Myers, and D.J. Lipman. 1990. Basic local alignment search tool. Journal of Molecular Biology, 215: 403–410. doi: 10.1016/S0022-2836(05)80360-2
Álvarez, L.R. 2003. Los manglares de Colombia y la recuperación de sus áreas degradadas: revisión bibliográfica y nuevas experiencias. Madera y Bosques 9: 3–25. doi: 10.21829/myb.2003.911286
Arnold, A.E. 2007. Understanding the diversity of foliar endophytic fungi: progress, challenges, and frontiers. Fungal Biology Reviews, 21: 51–66. doi: 10.1016/j.fbr.2007.05.003
Aveskamp, M.M., J. De Gruyter, and P.W. Crous. 2008. Biology and recent developments in the systematics of Phoma, a complex genus of major quarantine significance. Fungal Diversity, 31: 1–18. Avaliable at: https://www.fungaldiversity.org/fdp/sfdp/31-1.pdf
Aveskamp, M.M., G. Verkley, J. De Gruyter, M.A. Murace, A. Perelló, J.H.C. Woudenberg, J.Z. Groenewald, and P.W. Crous. 2009. DNA phylogeny reveals polyphyly of Phoma section Peyronellaea and multiple taxonomic novelties. Mycologia, 101: 363–382. doi: 10.3852/08-199
Begoude, A., B. Slippers, M. Wingfeld J. Roux. 2010. Botryosphaeriaceae associated with Terminalia catappa in Cameroon, South Africa and Madagascar. Mycological Progress, 9: 101–123. doi: 10.1007/s11557-009-0622-4
Bernal, R., S.R. Gradstein, and M. Celis. 2015. Catálogo de plantas y líquenes de Colombia, Mora oleifera (Hemsl.) Ducke. Instituto de Ciencias Naturales, Universidad Nacional de Colombia, Bogotá. Avaliable at: http://catalogoplantasdecolombia.unal.edu.co
Bolívar-Anillo, H.J., A.Z. Visbal, M.C. Serrano, H.S. Moreno, and D.A.V. Daza. 2020. A Preliminary Review on the Importance of Colombian Mangroves as a Source of Endophytic Microorganisms Relevant in Pharmaceutical Industry. Journal of Acupuncture & Traditional Medicine, 3, 006
Burgess, T.I., P.A. Barber, S. Mohali, G. Pegg, W. de Beer, and M.J. Wingfield. 2006. Three new Lasiodiplodia spp. from the tropics, recognized based on DNA sequence comparisons and morphology. Mycologia, 98: 423–435. doi: 10.1080/15572536.2006.11832677
Chen, Q., J.R. Jiang, G.Z. Zhang, L. Cai, and P.W. Crous. 2015a. Resolving the Phoma enigma. Stud. Mycol. 82: 137–217. doi: 10.1016/j.simyco.2015.10.003
Chen, Q., K. Zhang, G.Z. Zhang, and L. Cai. 2015b. A polyphasic approach to characterise two novel species of Phoma (Didymellaceae) from China. Phytotaxa 197: 267–281. https://doi.org/10.11646/phytotaxa.197.4.4
Coutinho, I., F., Freire, C. Lima, J. Lima, F. Gonçalves, A. Machado, A. Silva, and J. Cardoso. 2017. Diversity of genus Lasiodiplodia associated with perennial tropical fruit plants in northeastern Brazil. Plant Pathology, 66: 90–104. doi: 10.1111/ppa.12565
Cornejo, X., E. Peña, J. Cantera, P. Silverstone, R. Linares, M. Monzón, and C. Bonifaz. 2014. Mangrove forest of the Pacific coast of Colombia. In: Plants of South American Pacific Mangrove Swamps (Colombia, Ecuador, Peru). pp 12–20.
Darriba, D., G. Taboada, R. Doallo, and D. Posada. 2012. jModelTest2: more models, new heuristics and parallel computing. Nature Methods, 9. doi: 10.1038/nmeth.2109
Demers, D.H., M.A. Knestrick, R. Fleeman, R. Tawfik, A. Azhari, et al. (2018). Exploitation of mangrove endophytic fungi for infectious disease drug discovery. Mar Drugs 16. doi: 10.3390/md16100376
De Souza, S.F.L., A.S.R. Dumaresq, P.T. Lacava, R. Harakava, J.L. Azevedo, I.S. de Melo, and A. Pizzirani. 2013. Species diversity of culturable endophytic fungi from Brazilian mangrove forests. Current Genetics, 59: 153–166. doi: 10.1007/s00294-013-0396-8
El-Sayed, ER., A.S. Ahmed, and H.K. Abdel-hakim. 2020. A novel source of the cardiac glycoside digoxin from the endophytic fungus Epicoccum nigrum: isolation, characterization, production enhancement by gamma irradiation mutagenesis and anticancer activity evaluation. Journal of Applied Microbiology, 128: 747–762. doi: 10.1111/jam.14510
Favaro, L.C., F.L. De Melo, C.I. Aguilar, and W.L. Araújo. 2011. Polyphasic analysis of intraspecific diversity in Epicoccum nigrum warrants reclassification into separate species. PLoS One, 6. doi: 10.1371/journal.pone.0014828
Floudas, D, B.W. Held, R. Riley, L.G. Nagy, G. Koehler, A.S. Ransdell, H. Younus, J. Chow, J. Chiniquy, A. Lipzen, A. Tritt, H. Sun, S. Haridas, K. LaButti, R.A. Ohm, U. Kües, R.A. Blanchette, I.V. Grigoriev, R.E. Minto, and D.S. Hibbett. 2015. Evolution of novel wood decay mechanisms in Agaricales revealed by the genome sequences of Fistulina hepatica and Cylindrobasidium torrendii. Fungal Genetics and Biology, 76: 78–92. doi: 10.1016/j.fgb.2015.02.002
Galan, R., and J.T. Palmer. 2001. The occurrence of the rare Ciboria aestivalis in Europe. Czech Mycology, 52: 227–287. doi: 10.33585/cmy.52404
Gamboa-Gaitán, M.A., and J.T. Otero-Ospina. 2016. Colombian vanilla and its microbiota. III. Diversity and structure of the endophytic community. Acta Botanica Hungarica, 58: 241–256. doi: 10.1556/abot.58.2016.3-4.2
García, C. 2010. Diagnóstico de las áreas marinas y costeras protegidas, y de las áreas de manejo en el Pacífico colombiano. Fundación MarViva, Colombia, pp 65. Avaliable at: https://www.researchgate.net/publication/323656733
Gilbert, G.S., M. Mejia, and E. Rojas. 2002. Fungal diversity and plant disease in mangrove forests: salt excretion as a possible defense mechanism. Oecologia, 132: 278–285. doi: 10.1007/s00442-002-0966-9
Giri, C., E. Ochieng, L.L. Tieszen, Z. Zhu, A. Singh, T. Loveland, J. Masek, N. Duke. 2011. Status and distribution of mangrove forests of the world using earth observation satellite data. Global Ecology and Biogeography, 20: 154–159. doi: 10.1111/j.1466-8238.2010.00584.x
Gomes, A.R.P., and F. Wartchow. 2014. Coprinellus arenicola, a new species from Paraíba, Brazil. Sydowia, 66: 249–256. doi: 10.12905/0380.sydowia66(2)2014-0249
Gonda, S., A. Kiss-Szikszai, Z. Szucs, B. Balla, and G. Vasas. 2016. Efficient biotransformation of non-steroid anti-inflammatory drugs by endophytic and epiphytic fungi from dried leaves of a medicinal plant, Plantago lanceolata L. International Biodeterioration & Biodegradation, 108: 115–121. doi: 10.1016/j.ibiod.2015.12.018
Guindon, S., and O. Gascuel. 2003. A simple, fast and accurate method to estimate large phylogenies by maximum-likelihood. Systematic Biology, 52: 696–704. doi: 10.1080/10635150390235520
Guo, H., B. Sun, H. Gao, X. Chen, S. Liu, X. Yao, X. Liu, and Y. Che. 2009. Diketopiperazines from the Cordyceps-colonizing fungus Epicoccum nigrum. Journal of Natural Products, 72: 2115–2119. doi: 10.1021/np900654a
Harwoko, H., G. Daletos, F. Stuhldreier, J. Lee, S. Wesselborg, M. Feldbrügge, W.E.G. Müller, R. Kalscheuer, E. Ancheeva, and P. Proksch. 2019. Dithiodiketopiperazine derivatives from endophytic fungi Trichoderma harzianum and Epicoccum nigrum. Natural Product Research, 18: 1–9. doi: 10.1080/14786419.2019.1627348
Hibbett, D.S., A. Ohman, D. Glotzer, M. Nuhn, P. Kirk, and R.H. Nilsson. 2011. Progress in molecular and morphological taxon discovery in Fungi and options for formal classification of environmental sequences. Fungal Biology Reviews, 25: 38–47. doi: 10.1016/j.fbr.2011.01.001
Hussain, S., M. Usman, N-ul-S. Afshan, H. Ahmad, J. Khan, and A.N. Khalid. 2018. The genus Coprinellus (Basidiomycota; Agaricales) in Pakistan with the description of four new species. MycoKeys, 39: 41–61. doi: 10.3897/mycokeys.39.26743
Hyde, K.D., and S.Y. Lee. 1995. Ecology of mangrove fungi and their role in nutrient cycling – what gaps occur in our knowledge. Hydrobiologia, 295: 107–118. doi: 10.1007/BF00029117
Hyde, K.D., and E.B.G. Jones. 1988. Marine mangrove fungi. P.S.Z.N.I. Marine Ecology, 9: 15–33. doi: 10.1111/j.1439-0485.1988.tb00196.x
Hyde, K.D., R.H., Nilsson, S.A. Alias, H.A. Ariyawansa, J.E. Blair, L. Cai, A.W.A.M de Cock, A.J. Dissanayake, S.L. Glockling, I.D. Goonasekara, M. Gorczak, M. Hahn, R.S. Jayawardena, J.A.L. Kan. M.H. Laurence, C.A. Lévesque, X. Li, J.K. Liu, S.S.N. Maharachchikumbura, D.S. Manamgoda, F.N. Martin, E.H.C. McKenzie, A.R. McTaggart, P.E. Mortimer, P.V.R. Nair, J. Pawłowska, T.L. Rintoul, R.G. Shivas, C.F.J. Spies, B.A. Summerell, P.W.J. Taylo, R.B. Terhem, D. Udayanga, N. Vaghefi, G. Walther, M. Wilk, M. Wrzosek, J,C. Xu, J.Y. Yan, and N. Zhou. 2014. One stop shop: backbones trees for important phytopathogenic 5 genera: I. Fungal Diversity, 67: 21–125. doi: 10.1007/s13225-014-0298-1
Joel, E.L., and B.V. Bhimba. 2013. Biological activity of secondary metabolites isolated from mangrove fungi Neurospora crassa. Journal of Environmental Biology, 34: 729–32
Jones, E.B.G., and T.K. Tan. 1987. Observations on manglicolous fungi from Malaysia. Transactions of the British Mycological Society, 89: 390–392. doi: 10.1007/BF00029116
Katoh, K., J., Rozewicki, and K.D. Yamada. 2019. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics, 20: 1160–1166. doi: 10.1093/bib/bbx108
Kirk PM, P.F. Cannon, D.W. Minter, and J.A. Stalpers. 2008. Dictionary of the Fungi, 10th edn. CABI, Wallingford.
Kumar, S., G. Stecher, M. Li, C. Knyaz, and K. Tamura. 2018. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Molecular Biology and Evolution, 35: 1547–1549. doi: 10.1093/molbev/msy096
Kuraku, S., C.M. Zmasek, O. Nishimura, and K. Katoh. 2013. Leaves facilitates on-demand exploration of metazoan gene family trees on MAFFT sequence alignment server with enhanced interactivity. Nucleic Acids Research, 41: W22–W28. doi: 10.1093/nar/gkt389
López, A., C. Roberts, A. Tilley, J. Hawkins, and R. Cooke. 2016. Mangroves and people: Lessons from a history of use and abuse in four Latin American countries. Forest Ecology and Management, 368: 151–162. doi: 10.1016/j.foreco.2016.03.020
Lu, X., X. Wang, L. Zhang, X. Li, and X. Qi. 2020. Rare Fungal Keratitis Caused by Coprinellus Radians. Mycopathologia, 18: 389–394. doi: 10.1007/s11046-019-00414-y
Manamgoda, D.S., L. Cai, A.H. Bahkali, E. Chukeatirote, and K.D. Hyde. 2011. Cochliobolus: an overview and current status of species. Fungal Diversity, 51: 3–42. doi: 10.1007/s13225-011-0139-4
Mejía, Q., J. Molina, M. Sanjuan, B. Grijalba, and M. Niño. 2014. Bosque de manglar, un ecosistema que debemos cuidar. Universidad Jorge Tadeo Lozano, Instituto Colombiano de Desarrollo Rural, Cartagena DT, pp 27
Mehl, J., M.J. Wingfield, J. Roux, and B. Slippers. 2017. Invasive everywhere? Phylogeographic analysis of the globally distributed tree pathogen Lasiodiplodia theobromae. Forests 8. doi: 10.3390/f8050145
Misral, D.S. 2002. Development of Mixed Formation of Fungal. (Trichoderma) and bacterial (pseudomonas) Biocontrol Agents for Management of Plant Disease. Ph.D Thesis Submitted to G.B Part University of Agriculture and Technology Pantnager, 185 pp 65.
Mohali, S.R., F. Castro, J.R. Úrbez, and W.D. Gubler. 2017. First report of Lasiodiplodia theobromae and L. venezuelensis associated with blue stain on Ficus insipida wood from the Natural Forest of Venezuela. c 47: 1–5. doi: 10.1111/efp.12355
Mohapatra. 2008. Textbook of Environmental Microbiology. I K International Publishing, New Delhi
Munkvold, G.P. 2017. Fusarium Species and Their Associated Mycotoxins. Methods in Molecular Biology, l 1542: 51–106. doi: 10.1007/978-1-4939-6707-0_4
Nagy, L.G., S. Kocsubé, Z. Csanádi, G.M. Kovács, T. Petkovits, C. Vágvölgyi, and T. Papp. 2012. Re-Mind the Gap! Insertion - Deletion Data Reveal Neglected Phylogenetic Potential of the Nuclear Ribosomal Internal Transcribed Spacer (ITS) of Fungi. PLoS One 7. doi: 10.1371/journal.pone.0049794
Ordóez, N.F., J.T. Otero, and M.C. Díez. 2012. Hongos endófitos de orquídeas y su efecto sobre el crecimiento en Vanilla planifolia Andrews. Acta Agron 61: 282–290. Avaliable at: https://revistas.unal.edu.co/index.php/acta_agronomica/article/view/37544/39920
Osorio, J.A., C.J. Crous, Z.W. De Beer, M.J. Wingfield, and J. Roux. 2017. Endophytic Botryosphaeriaceae, including five new species, associated with mangrove trees in South Africa. Fungal Biology, 121: 361–393. doi: 10.1016/j.funbio.2016.09.004
Pal, A.K., and R.P. Purkayastha. 1992. New parasitic fungi from Indian mangrove. Journal of Mycopathological Research, 30: 173–176.
Palacios, M.L., and J.R. Cantera. 2017. Mangrove timber use as an ecosystem service in the Colombian Pacific. Hydrobiologia 803: 345–358. doi: 10.1007/s10750-017-3309-x
Pavlic, D., B. Slippers, T.A. Coutinho, and M.J. Wingfield. (2007). Botryosphaeriaceae occurring on native Syzygium cordatum in South Africa and their potential threat to Eucalyptus. Plant Patholology Journal, 56: 624–636. doi: 10.1111/j.1365-3059.2007.01608.x
Perveen, I., M.A. Raza, T. Iqbal, I. Naz, S. Sehar, and S. Ahmed. 2017. Isolation of anticancer and antimicrobial metabolites from Epicoccum nigrum; endophyte of Ferula sumbul. Microbial Pathogenesis, 110: 214–224. doi: 10.1016/j.micpath.2017.06.033
Petrini, O. 1991. Fungal Endophytes of Tree Leaves. En: Andrews JH, Hirano SS (eds) Microbial Ecology of Leaves. Brock/Springer Series in Contemporary Bioscience, Springer, New York, NY. doi: 10.1007/978-1-4612-3168-4_9
Phillips, A.J.L., A. Alves, J. Abdollahzadeh, B. Slippers, M.J. Wingfield, J.Z. Groenewald, and P.W. Crous. 2013. The Botryosphaeriaceae: genera and species known from culture, Studies in Mycology, 76: 51–167. doi: 10.3114/sim0021
Prahl, H.V. 1989. Manglares de Colombia. Villegas (Ed.), Banco de Occidente, pp 205
Punithalingam, E. 1980. Plant Diseases Attributed to Botryodiplodia theobromae Pat. Vadus, Germany
Raeder, U., and P. Broda. 1985. Rapid preparation of DNA from filamentous fungi. Letters in Applied Microbiology, 1: 17–20. doi: 10.1111/j.1472-765X.1985.tb01479.x
Rambaut A, A.J. Drummond, D. Xie, G. Baele, and M.A. Suchard. 2018. Posterior Summarization in Bayesian Phylogenetics Using Tracer 1.7. Systematic Biology, 67: 901–904. doi: 10.1093/sysbio/syy032
Rhim, H., J.Y. Park, D.J. Lee, and J.I. Han. 2019. Epicoccum nigrum-induced respiratory infection in a wild Eurasian scops owl (Otus scops). Journal of Veterinary Medical Science, 81: 1348–1350. doi: 10.1292/jvms.19-0172
Rineau, F., F. Shah, M.M. Smits, P. Persson, T. Johansson, R. Carleer, C. Troein, and A. Tunlid. 2013. Carbon availability triggers the decomposition of plant litter and assimilation of nitrogen by an ectomycorrhizal fungus. ISME J. 7. doi: 10.1038/ismej.2013.91
Ronquist, F., M. Teslenko, P. van der Mark, D.L. Ayres, A. Darling, S. Höhna, B. Larget, L. Liu, M.A. Suchard, and J.P. Huelsenbeck. 2012. MRBAYES 3.2: Efficient Bayesian phylogenetic inference and model selection across a large model space. Systematic Biology, 61: 539–542. doi: 10.1093/sysbio/sys029
Sakalidis, M.L., G.E.S.J. Hardy, and T.I. Burgess. 2011. Class III endophytes, clandestine movement amongst hosts and habitats and their potential for disease; a focus on Neofusicoccum australe. Australasian Plant Pathology, 40: 510–521. doi: 10.1007/s13313-011-0077-3
Salazar, C.S., and M.C.C. De García. 2005. Aislamiento de hongos endófitos en rosa (Rosa hybrida) en Bogotá, Colombia, Revista Iberoamericana de Micología, 22: 99–101. doi: 10.1016/S1130-1406(05)70016-4
Schafer, D.J. 2010. Keys to sections of Parasola, Coprinellus, Coprinopsis and Coprinus in Britain. Field Mycology, 11: 44–51. doi: 10.1016/j.fldmyc.2010.04.006
Shearer, C.A., E. Descals, B. Kohlmeyer, J. Kohlmeyer, L. Marvanová, D. Padgett, D. Porter, H.A. Raja, J.P. Schmit, H.A. Thorton, and H. Voglymayr. 2007. Fungal biodiversity in aquatic habitats. Biodiversity and Conservation, 16: 49–67. doi: 10.1007/s10531-006-9120-z
Shetty, K.G., A.M. Minnis, Rossman, and A.Y., Jayachandran, K. (2011). The Brazilian peppertree seed-borne pathogen, Neofusicoccum batangarum, a potential biocontrol agent. Biological Control, 56, 91–97. doi: 10.1016/j.biocontrol.2010.09.016
Simões, M.F., A. Antunes, C.A. Ottoni, M.S. Amini, I. Alam, H. Alzubaidy, N.A. Mokhtar, J.A.C. Archer, and V.B. Bajic. 2015. Soil and rhizosphere associated fungi in gray mangroves (Avicennia marina) from the Red Seada metagenomic approach. Genomics, Proteomics & Bioinformatics, 13: 310–320. doi: 10.1016/j.gpb.2015.07.002
Singh, L.P., S.S. Gill, and N. Tuteja. 2011. Unraveling the role of fungal symbionts in plant abiotic stress tolerance. Plant Signaling & Behavior, 6: 175–191. doi: 10.4161/psb.6.2.14146
Sivanesan, A. 1984. The bitunicate ascomycetes and their anamorphs. J. Cramer, Vaduz.
Slippers, B., B.A. Summerell, P.W. Crous, T.A. Coutinho, B.D. Wingfield, and M.J. Wingfield. 2005. Preliminary studies on Botryosphaeria species from Southern Hemisphere conifers in Australasia and South Africa. Australasian Plant Pathology, 34: 213–220. doi: 10.1071/AP05020
Slippers, B., and M.J. Wingfield. 2007. Botryosphaeriaceae as endophytes and latent pathogens of woody plants: diversity, ecology and impact. Fungal Biology Reviews, 21: 90–106. doi: 10.1016/j.fbr.2007.06.002
Spalding, M., M. Kainuma, and L. Collins. 2010. World Atlas of Mangroves. Earthscan, London, pp 336
Suetrong, S., S. Preedanon, A. Klaysuban, W. Gundool, P. Unagul, J. Sakayaroj, W. Promchu, and T. Sangtiean. 2017. Distribution and occurrence of manglicolous marine fungi from eastern and southern Thailand. Botanica Marina, 60: 503–514. doi: 10.1515/bot-2016-0107
Swofford, D.L. 2003. PAUP*: phylogenetic analysis using parsimony (*and other methods). Sinauer Associates, Sunderland, Massachusetts.
Thamizhmani, R., and R. Senthilkumaran. 2012. Diversity of fungi in selected mangroves along the east coast of India. International Journal of Current Microbiology and Applied Sciences, 1: 29–33
Thatoi, H., B.C. Behera, R.R. Mishra, and S.K. Dutta. 2013. Biodiversity and biotechnological potential of microorganisms from mangrove ecosystems: a review. Annals of Microbiology 63: 1–19. doi:10.1007/s13213-012-0442-7
Tomlinson, P. 2016. The Botany of Mangroves (2nd ed.). Cambridge: Cambridge University Press. doi: 10.1017/CBO9781139946575
Turner, B.C., D.D. Perkins, and A. Fairfield. 2001. Neurospora from natural populations: a global study. Fungal Genetics and Biology, 32: 67–92. doi: 10.1006/fgbi.2001.1247
Ukoima, H.N. 1996. Studies on Fungi Associated with some Mangrove Forest Trees in Rivers State. Ph.D Thesis. Rivers State University of Science and Technology, Nigeria.
Ukoima, H.N., and M.A. Amakiri. 2000. Fungi associated with the roots of Rhizophora mangle, Rhizophora harrisonii, and Avicennia Africana in Port Harcourt Mangrove swamp, Rivers State. Ecology, Environment and Conservation Journal, 13: 32–42.
Ukoima, H.N., M. Ikata, and G.A. Pepple. 2013. Control of Lasiodiplodia theobromae (PAT) on Rhizophora racemosa using plants extracts. American Journal of Biotechnology and Molecular Sciences, i 3: 1–7. doi: 10.5251/ajbms.2013.3.1.1.7
Ulloa, C., P. Acevedo, S. Beck, M.J. Belgrano, R. Bernal, P.E. Berry, L. Brako, M. Celis, G. Davidse, R.C. Forzza, S.R. Gradstein, O. Hokche, B. León, S. León, R.E. Magill, D.A. Neill, M.H. Nee, P.H. Raven, H. Stimmel, M.T. Strong, J.L. Villaseñor, J.L. Zarucchi, F.O. Zuloaga, and M.P Jørgensen. 2017. An integrated assessment of vascular plants species of the Americas. Science, 358: 1614–1617. doi: 10.1126/science.aao0398
Vázquez, P., G. Holguin, M.E. Puerte, A. Lopez-Cortes, and Y. Bashan. 2000. Phosphate-solubillising microorganisms associated with the rhizosphere of mangroves in a semiarid Coastal lagoon. Biology and Fertility of Soils, 30: 460–46. doi: 10.1007/s003740050024
Vega, F.E., A. Simpkins, M.C. Aime, F. Posada, S.W. Peterson, S.A. Rehner,... and A.E. Arnold. 2010. Fungal endophyte diversity in coffee plants from Colombia, Hawai, Mexico and Puerto Rico. Fungal Ecology, 3: 122-138. doi: 10.1016/j.funeco.2009.07.002
Vittal, B.P.R., and V.V. Sarma. 2006. Diversity and ecology of fungi on mangroves of bay of Bengal region – an overview. Indian Journal of Geo-Marine Sciences, 35: 308–317
Wang, J.M., G.Z. Ding, L. Fang, J.G. Dai, S.S. Yu, Y.H. Wang, X.G. Chen, S.G. Ma, J. Qu, S. Xu, and D. Du. 2010. Thiodiketopiperazines produced by the endophytic fungus Epicoccum nigrum. Journal of Natural Products, 73: 1240–1249. doi: 10.1021/np1000895
White, T. J., T. Bruns, S. Lee, and J. Taylor. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: M. A. Innis, D. H. Gelfand, J. J. Sninsky, & T. J. White (Eds.), PCR protocols: a guide to methods and applications (pp. 315–322). Academic Press.Wilson D (1995) Endophyte: The evolution of a term, and clarification of its use and definition. Oikos, 73: 272–276. doi: 10.2307/3545919
Xing, X.K., J. Chen, M.J. Xu, W.H. Lin, and S.X. Guo. 2011. Fungal endophytes associated with Sonneratia (Sonneratiaceae) mangrove plants on the south coast of China. Forest Pathology, 41: 334–340. doi: 10.1111/j.1439-0329.2010.00683.x
Yan, Z., S. Wen, M. Ding, H. Guo, C. Huang, X. Zhu, J. Huang, Z. She, and Y. Long. 2019. The Purification, Characterization, and Biological Activity of New Polyketides from Mangrove-Derived Endophytic Fungus Epicoccum nigrum SCNU-F0002. Marine Drugs, 17. doi: 10.3390/md17070414
Descargas
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2023 jhon alexander osorio

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-CompartirIgual 4.0.

